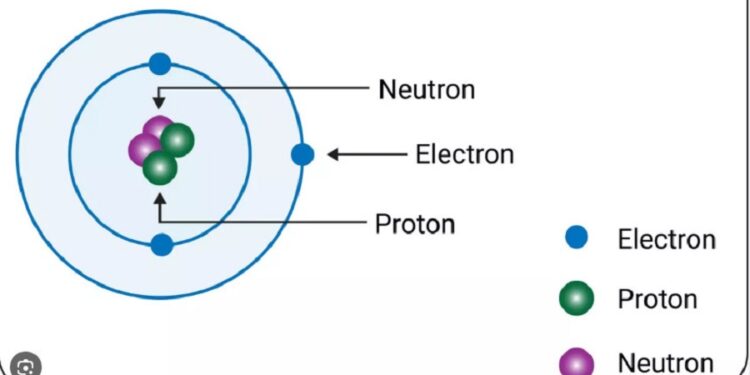
The discovery of neutrons, which possess no electric charge and nearly the same mass as protons while remaining electrically neutral, was originally proposed by J.J Thomson in 1903.
The fundamental constituents of matter are subatomic particles, with the atom serving as the smallest unit. Comprising protons, neutrons, and electrons, atoms possess protons and neutrons in the nucleus at the center, while electrons revolve around the nucleus in specific orbits. These subatomic particles were uncovered by investigators in the nineteenth and twentieth centuries. Significantly, in 1932, Sir James Chadwick, a British physicist, discovered the neutron, bringing a noteworthy advance in the understanding of atomic structure. In recognition of his achievement, he was awarded the Nobel Prize in Physics in 1935.
Table of Contents
ToggleDiscovery of Protons
In 1815, William Prout, an English chemist, proposed that all atoms were composed of hydrogen atoms, or “protyles.” The discovery of canal rays, which are positively charged ions formed by gases, occurred in 1886 by the German physicist Eugen Goldstein. Through observation, it was noted that among ionized gases, the hydrogen ion possessed the highest charge-to-mass ratio and the smallest size. These findings contributed to the discovery of protons.

Ernest Rutherford famously discovered the nucleus of the atom during his gold foil experiment in 1911. From his findings, he concluded that the positively charged particles within an atom were concentrated in a singular core, whilst the majority of the atom’s volume was empty. Additionally, he determined that the total number of positively charged particles within the nucleus equaled the total number of negatively charged electrons orbiting around it.
Maybe you are interested: Who created the first atomic bomb formula?
Who Discovered Protons?
The discovery of the proton is credited to Ernest Rutherford, who proved that the nucleus of the hydrogen atom (i.e. a proton) is present in the nuclei of all other atoms in the year 1917.

With regards to the findings derived from the gold-foil experiment, Rutherford is recognized for the identification of the atomic nucleus.
How was the Proton Discovered?
- Ernest Rutherford observed that his scintillation detectors detected hydrogen nuclei when a beam of alpha particles was shot into the air.
- After investigating further, Rutherford found that these hydrogen nuclei were produced from the nitrogen atoms present in the atmosphere.
- He then proceeded to fire beams of alpha particles into pure nitrogen gas and observed that a greater number of hydrogen nuclei were produced.
- He concluded that the hydrogen nuclei originated from the nitrogen atom, proving that the hydrogen nucleus was a part of all other atoms.
- This experiment was the first to report a nuclear reaction, given by the equation: 14N + α → 17O + p [Where α is an alpha particle which contains two protons and two neutrons, and ‘p’ is a proton]
- The hydrogen nucleus was later named ‘proton’ and recognized as one of the building blocks of the atomic nucleus.
Maybe you are interested: Who is the painter of The Mona Lisa?
Discovery of Neutrons
In the year 1930, Herbert Becker and Walther Bothe, German nuclear physicists, made the pivotal observation that a type of penetrating radiation was generated when alpha particles discharged by polonium came in contact with lighter elements including beryllium, lithium, and boron. The radiation, impervious to electric fields, was established as gamma radiation, leading to the discovery of neutrons.
During 1932, Frederic Joliot-Curie and Irene Joliot-Curie, French scientists, made an observation regarding the impact of unusually penetrating radiation on paraffin wax or other hydrogen-rich compounds. This led to the ejection of high energy protons, measuring approximately 5 MeV. Ettore Majorana, an Italian physicist, proposed the presence of a neutral particle in the atomic nucleus that prompted the interaction of radiation with protons.
In 1920, Ernest Rutherford proposed the existence of neutral particles within the nuclei of atoms. He postulated that these particles were comprised of a bound proton and electron, resulting in a net neutral charge. To designate these particles, Rutherford introduced the term “neutron.”
Who Discovered Neutrons?
The British physicist Sir James Chadwick discovered neutrons in the year 1932. He was awarded the Nobel Prize in Physics in the year 1935 for this discovery.

Mr. Ernest Rutherford’s theoretical proposal of the neutron in 1920 holds significant importance and deserves attention.
How were Neutrons Discovered?
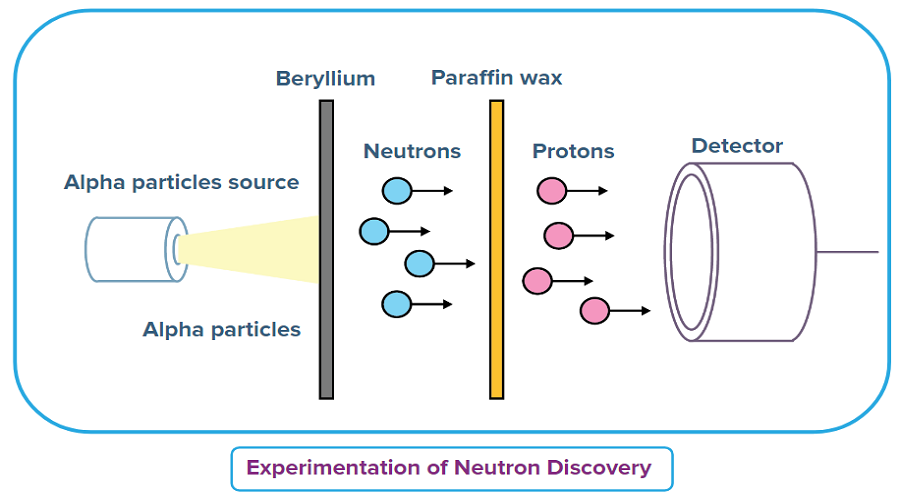
- James Chadwick fired alpha radiation at beryllium sheet from a polonium source. This led to the production of an uncharged, penetrating radiation.
- This radiation was made incident on paraffin wax, a hydrocarbon having a relatively high hydrogen content.
- The protons ejected from the paraffin wax (when struck by the uncharged radiation) were observed with the help of an ionization chamber.
- The range of the liberated protons was measured and the interaction between the uncharged radiation and the atoms of several gases was studied by Chadwick.
- He concluded that the unusually penetrating radiation consisted of uncharged particles having (approximately) the same mass as a proton. These particles were later termed ‘neutrons’.
Frequently Asked Questions – FAQs
Who created the first atomic theory?
In the ancient times, the atomic hypothesis was first introduced by the Greek philosophers Leucippus and Democritus during the 5th century BC, while the Roman philosopher and poet Lucretius revived the theory in the 1st century BC.
Who named the atom?
The term “atom” finds its origins in ancient Greece, around 400 B.C., to the time of the philosopher Democritus. He coined the Greek word “atomos,” which translates to “uncuttable.” According to Democritus, all matter can be broken down into small, distinct particles referred to as atomos.
What is the smallest subatomic particle?
According to current scientific understanding, quarks are considered the most minute subatomic particles. In fact, they have been identified as essential constituents of all modern elementary particles, serving as the fundamental building blocks of matter. This supersedes the traditional belief that protons, neutrons, and electrons are the cornerstone particles of the universe.
What is Dalton’s theory?
Dalton’s atomic theory was a seminal attempt to comprehensively illustrate all matter by means of atoms and their properties. The primary aspect of his theory maintains that all matter is comprised of indivisible atoms. Furthermore, Dalton’s theory’s secondary component posits that all atoms of a specific element possess the same characteristics and mass.
What is the failure of Dalton’s atomic theory?
Dalton’s atomic theory could not account for the differences in characteristics between various allotropes of the same element. To create compounds, elements must mix in simple, whole-number ratios, according to this hypothesis. However, this isn’t always the case.
What is a proton?
What is a neutron?
Who discovered protons?
Who discovered neutrons?
Why do neutrons have no charge?
Who discovered electrons protons and neutrons?
The discovery of neutrons, which possess no electric charge and nearly the same mass as protons while remaining electrically neutral,...
Read moreWho is the painter of The Mona Lisa?
The Louvre Museum houses one of the most iconic portraits in the history of art, namely the Mona Lisa painting....
Read moreWho makes Genesis vehicles?
The Hyundai Motor Group produces luxurious automobiles under the name of Genesis. In 2015, the company announced its intention to...
Read moreWho discovered United States of America?
The response to the aforementioned inquiry appears to be apparent for the majority of individuals, as they were educated in...
Read moreWho created the first atomic bomb formula?
The journey towards the creation of the atomic bomb was initiated by a moment of profound realization in London. Subsequently,...
Read moreWho has the most followers on instagram in the world?
In response to an inquiry of utmost interest, it has been revealed that the individual with the most followers on...
Read moreQuotes about friends who are fake
The end of a friendship can be an excruciatingly painful experience, potentially overshadowing other kinds of heartbreak. The betrayal of...
Read moreWho is the top 10 richest people in Asia?
The ever-changing wealth landscape in Asia has continually garnered international attention, as a vast number of individuals have left their...
Read moreWho is the most richest person in the world currently?
With a net worth of $239.3B, Elon Musk currently holds the top position on the list of the world's wealthiest...
Read moreHow deep is the wreck of The Titanic?
On April 15, 1912, the RMS Titanic endured a tragic fate in the North Atlantic Ocean as it collided with...
Read moreThe biggest country in the world by population
Data published by the World Bank reveals that in 2021, the 25 most populous countries in the world accounted for...
Read more